ISO Certifications for Veterinary Pharmaceutical Manufacturing Businesses, Requirements and Benefits

Introduction to ISO Standards for Veterinary Pharmaceutical Manufacturing
The veterinary pharmaceutical manufacturing sector is essential for safeguarding animal health, which directly impacts food safety and agricultural economies. In this context, adherence to globally recognized ISO standards is not just a regulatory necessity but a hallmark of quality and trustworthiness. ISO certifications for veterinary pharmaceutical manufacturing ensure products meet stringent safety, quality, and ethical standards.
The rising demand for veterinary products, especially in the post-pandemic era, has brought a sharper focus on compliance and quality assurance. ISO certifications provide a structured framework for managing operations, enhancing product safety, and promoting sustainability within the veterinary pharmaceutical industry.
If you’re seeking ISO certification for veterinary pharmaceutical manufacturing, reach out to us today at [email protected] or call +91-8595603096 to get started.
Applicable ISO Standards for Veterinary Pharmaceutical Manufacturing
Understanding and implementing the appropriate ISO standards is vital for veterinary pharmaceutical manufacturers to achieve operational excellence and market credibility. Here’s a breakdown of the key ISO standards applicable to the industry:
ISO 9001: Quality Management Systems
ISO 9001 is the cornerstone of quality management, emphasizing customer satisfaction, process improvement, and operational efficiency. For veterinary pharmaceutical manufacturers, it ensures robust quality control mechanisms throughout the product lifecycle.
ISO 14001: Environmental Management Systems
This standard addresses the environmental impacts of manufacturing processes. Adopting ISO 14001 helps veterinary pharmaceutical companies minimize waste, reduce emissions, and adopt sustainable practices.
ISO 45001: Occupational Health and Safety Management Systems
ISO 45001 ensures a safe working environment for employees in manufacturing plants. By identifying potential hazards and mitigating risks, this standard promotes workplace safety and well-being.
ISO 17025: Testing and Calibration Laboratories
Veterinary pharmaceutical manufacturing involves rigorous testing of raw materials and finished products. ISO 17025 ensures that laboratories operate competently and produce reliable results, maintaining product efficacy and safety.
ISO 13485: Quality Management for Medical Devices
Though primarily designed for medical devices, ISO 13485 can be adapted for veterinary pharmaceutical manufacturing where precision and sterility are critical, such as injectable medications or implantable veterinary products.
ISO 26000: Social Responsibility
This standard emphasizes ethical operations and community engagement. For veterinary pharmaceutical manufacturers, ISO 26000 encourages practices that align with animal welfare, corporate social responsibility, and ethical sourcing.
Click here to find out more applicable standards to your industry
Pacific Certifications specializes in providing comprehensive ISO certification services for veterinary pharmaceutical manufacturers. With our expertise and industry-specific knowledge, we conduct audits and issue certifications that validate your organization’s compliance with global standards.
Our certification process ensures that your operations align with the latest ISO standards, fostering trust among stakeholders, enhancing regulatory compliance, and positioning your business for sustainable growth.
Ensure your veterinary pharmaceutical manufacturing meets global standards! Email us at [email protected] or call +91-8595603096 for expert ISO certification services.
Requirements of ISO Certifications for Veterinary Pharmaceutical Manufacturing
To achieve ISO certification, veterinary pharmaceutical manufacturers must adhere to a variety of requirements depending on the standard. Below are the core elements:
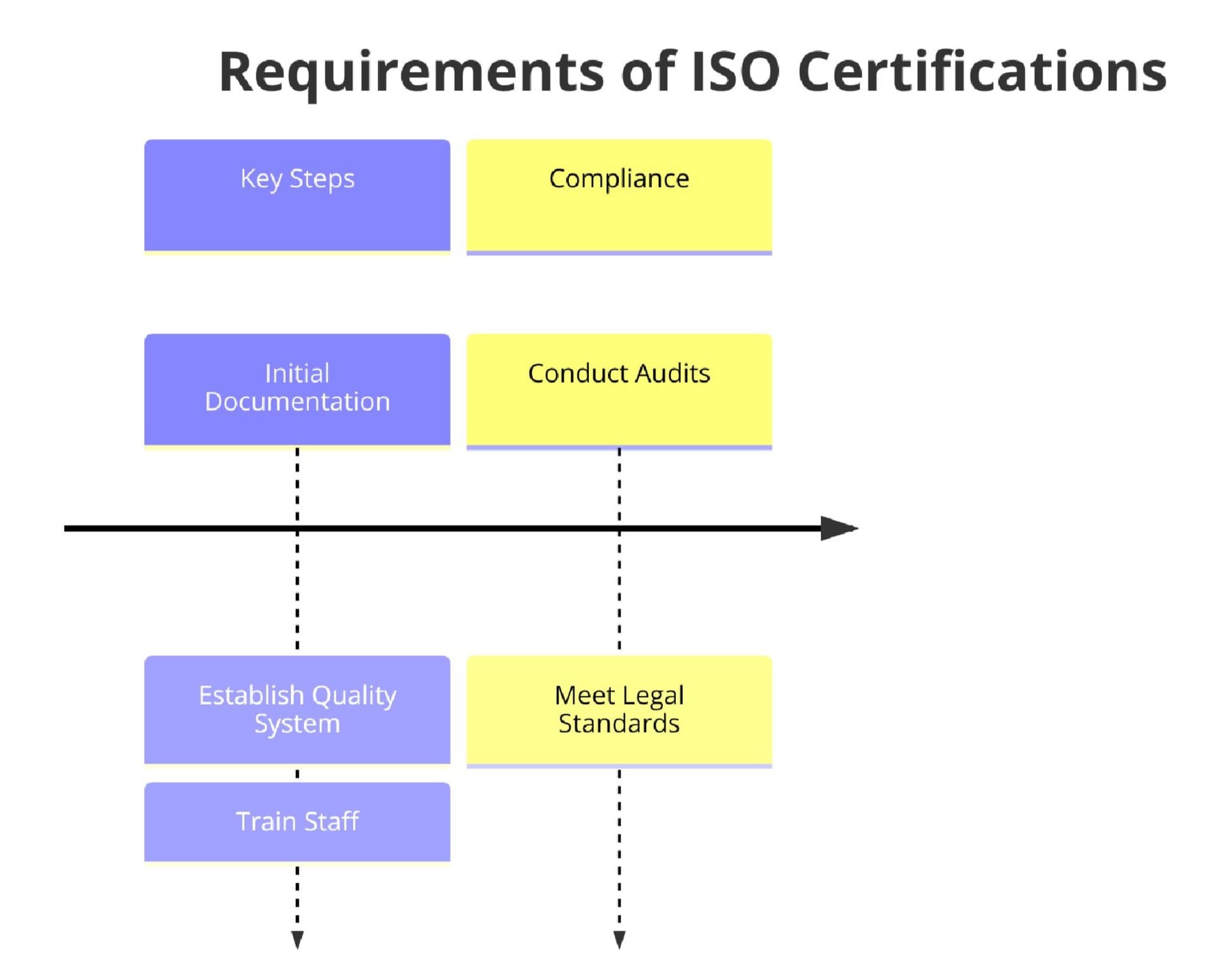
1. Quality Management Requirements (ISO 9001)
- Establishment of documented quality policies and objectives
- Implementation of process monitoring and control systems
- Regular internal audits and management reviews
2. Environmental Management Requirements (ISO 14001)
- Identification and mitigation of environmental impacts
- Compliance with environmental regulations
- Continuous improvement initiatives to reduce ecological footprints
3. Health and Safety Requirements (ISO 45001)
- Risk assessment and hazard identification
- Employee training and awareness programs
- Emergency preparedness and response mechanisms
4. Laboratory Accreditation Requirements (ISO 17025)
- Calibration and validation of testing equipment
- Competency of personnel conducting tests
- Detailed documentation and traceability of results
5. Ethical and Social Responsibility Requirements (ISO 26000)
- Adherence to ethical labor practices
- Transparency in business operations
- Engagement with community stakeholders
For reliable ISO certification for veterinary pharmaceutical manufacturing, connect with us at [email protected] or call +91-8595603096.
Benefits of ISO Certifications for Veterinary Pharmaceutical Manufacturing
ISO certifications bring a multitude of benefits, enabling veterinary pharmaceutical manufacturers to meet market demands while ensuring product quality and safety.
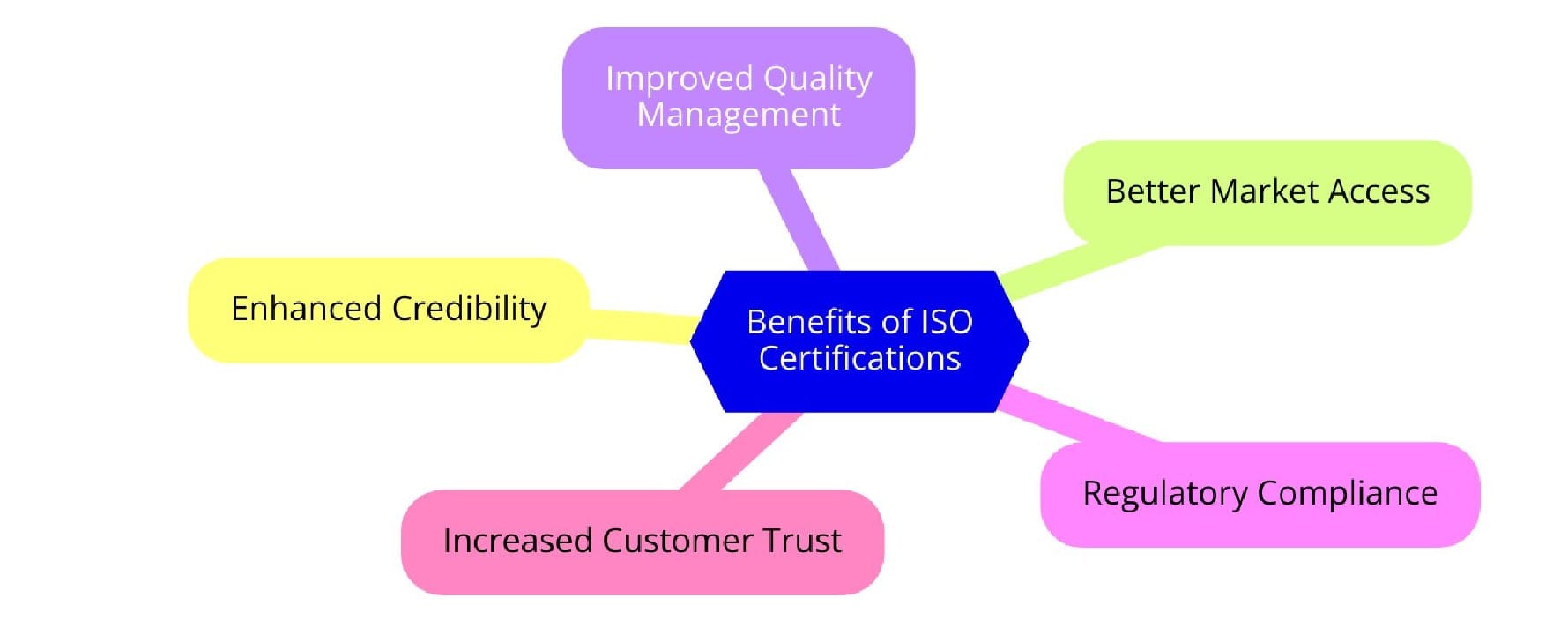
1. Enhanced Regulatory Compliance: ISO certifications ensure adherence to international regulations, reducing the risk of non-compliance and associated penalties.
2. Improved Product Quality: By standardizing processes, ISO certifications guarantee consistent product quality, which is critical in pharmaceuticals where variability can lead to adverse outcomes.
3. Increased Customer Confidence: Certification signals a commitment to quality and safety, strengthening customer trust and loyalty.
4. Cost Efficiency: Optimized processes and reduced wastage lead to significant cost savings over time.
5. Competitive Advantage: In a competitive market, ISO certifications distinguish manufacturers as reliable and compliant, opening doors to new markets and partnerships.
6. Environmental Sustainability: Adopting standards like ISO 14001 ensures manufacturers contribute positively to environmental conservation, a growing priority in 2024.
Ready to elevate your manufacturing operations with ISO certification? Contact Pacific Certifications at [email protected] or call +91-8595603096 for support.
Conclusion
In 2024, the veterinary pharmaceutical sector continues to evolve, driven by innovation and stricter regulations. The global market has witnessed a surge in demand for antibiotics, vaccines, and parasiticides, particularly for companion animals.
With increasing scrutiny on animal welfare and environmental sustainability, ISO standards have gained prominence as a benchmark for ethical and responsible manufacturing. Manufacturers investing in ISO certifications not only ensure compliance but also gain a competitive edge in a market that values transparency and sustainability.
Are you ready to elevate your veterinary pharmaceutical manufacturing operations to global standards? At Pacific Certifications, we simplify the ISO certification process, ensuring your organization meets and exceeds regulatory and market expectations.
Pacific Certifications is accredited by ABIS, in case you need support with ISO certification for your Veterinary Pharmaceutical Manufacturing business, please contact us at [email protected] or +91-8595603096.
Ready to get ISO certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –
Read more: Pacific Blogs

