ISO Certifications for Skincare Product Manufacturing Businesses, Requirements and Benefits

ISO Standards for Skincare Product Manufacturing
In the ever-evolving beauty and personal care industry, product safety, quality assurance & customer trust have taken center stage. As skincare becomes more science-driven and regulatory environments tighten globally, ISO certifications for skincare product manufacturing are not only valuable, they are essential. These internationally recognized standards establish trust in a brand and ensure regulatory compliance.
From formulation to packaging, ISO standards uphold a brand’s reputation while prioritizing consumer health. As industry demand for clean beauty and sustainable production rises, compliance with ISO requirements positions skincare manufacturers as responsible leaders in the marketplace.
The International Organization for Standardization develops globally recognized standards that serve as benchmarks for quality, safety, efficiency, and traceability. For the skincare sector, ISO standards are vital in aligning manufacturing operations with international best practices.
In a market defined by increasing scrutiny over ingredients and production methods, implementing to ISO standards signals credibility and readiness for global expansion.
Looking for ISO certification for your skincare manufacturing business?
Connect with us today at [email protected] or call us at +91-8595603096. Visit www.pacificcert.com to get started!
Applicable ISO Standards for Skincare Product Manufacturing
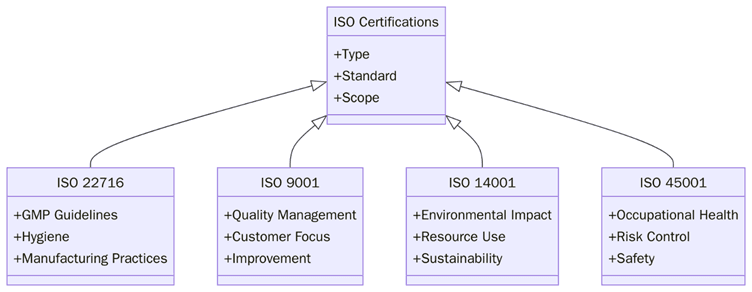
The skincare manufacturing industry is multifaceted, requiring a range of ISO standards tailored to different segments of the supply chain. Below are the most applicable ISO certifications:
ISO 22716 – Good Manufacturing Practices (GMP) for Cosmetics
This is the cornerstone ISO standard for skincare manufacturers. It outlines the requirements for GMP covering production, control, storage, and shipment of cosmetic products.
ISO 9001 – Quality Management Systems (QMS)
Applies to all types of manufacturing and service providers. It helps organizations consistently meet customer and regulatory requirements.
ISO 14001 – Environmental Management Systems
Guides organizations in reducing environmental impact. Sustainability-conscious skincare brands often pursue this to demonstrate environmental responsibility.
ISO 45001 – Occupational Health and Safety Management Systems
Ensures the health and safety of personnel involved in manufacturing.
ISO 16128 – Guidelines on Technical Definitions and Criteria for Natural and Organic Cosmetic Ingredients
Helps skincare brands validate natural and organic claims, increasingly important to eco-conscious consumers.
ISO 13485 – Quality Management Systems for Medical Devices
Relevant when skincare products border the medical/cosmeceutical realm, such as acne treatments or anti-aging serums regulated as medical devices in some jurisdictions.
Click here to find out more applicable standards to your industry
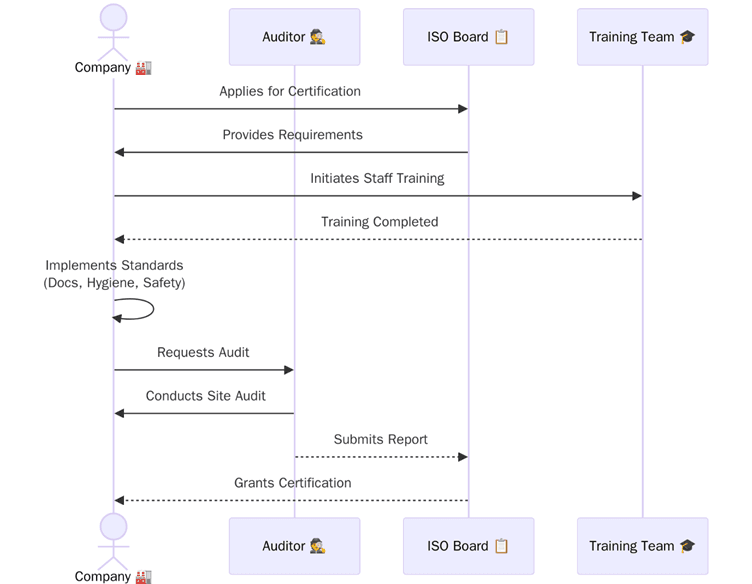
As a recognized certification body, Pacific Certifications plays a pivotal role in supporting skincare manufacturers to achieve ISO certification. Our team conducts third-party audits and issues ISO certificates that validate a company’s compliance with the required standard.
Although we do not provide consultancy, training, or implementation services, we ensure impartiality, transparency, and efficiency during the certification process. From application to audit and surveillance, we guide our clients through the audit lifecycle with strict adherence to ISO and IAF guidelines.
Ready to certify your skincare product manufacturing facility?
Email us at [email protected], dial +91-8595603096.
Requirements of ISO Certifications for Skincare Product Manufacturing
Below are the key requirements of each ISO standard relevant to skincare product manufacturing:
ISO 22716 – Good Manufacturing Practices (GMP) for Cosmetics
- Premises & Equipment: Must be hygienically designed and maintained.
- Personnel: Proper hygiene practices, training, and protective clothing.
- Raw Materials: Must be checked, labeled, and stored properly.
- Production: Controlled processes to prevent contamination.
- Finished Products: Proper storage, quality checks, and traceability.
- Documentation: SOPs and batch records must be maintained.
ISO 9001 – Quality Management Systems
- Customer Focus: Meeting customer and regulatory requirements.
- Leadership Commitment: Defined responsibilities and quality objectives.
- Process Approach: Clear workflows with continuous improvement.
- Risk Management: Identify and mitigate process risks.
- Performance Evaluation: Internal audits, customer feedback, data analysis.
- Improvement: CAPA (Corrective and Preventive Actions) to enhance systems.
ISO 14001 – Environmental Management Systems
- Environmental Policy: Commitment to sustainability and legal compliance.
- Impact Assessment: Identify and control environmental aspects.
- Legal Compliance: Meet all applicable environmental laws.
- Objectives & Targets: Define and track environmental goals.
- Operational Control: Waste management, energy use, emissions control.
- Monitoring: Regular review of environmental performance.
ISO 45001 – Occupational Health & Safety Management
- Hazard Identification: Recognize risks in the work environment.
- Legal Compliance: Adhere to health and safety laws.
- Worker Participation: Engage employees in safety programs.
- Emergency Preparedness: Plans for incidents and accidents.
- Monitoring: Regular safety audits and performance evaluations.
- Continuous Improvement: Eliminate hazards and reduce OH&S risks.
ISO 16128 – Natural & Organic Cosmetic Ingredients
- Ingredient Classification: Distinguishes natural, organic, derived natural, etc.
- Calculation Rules: Formulas for determining organic/natural content.
- Transparency: Accurate labeling based on quantitative assessments.
- Documentation: Evidence of natural/organic origin required.
ISO 13485 – Quality Management for Medical Devices (if applicable)
- Risk Management: From product design to delivery.
- Validation: Processes, including sterile and cleanroom operations.
- Traceability: Unique device identification and batch controls.
- Design Controls: For skincare products with therapeutic claims.
- Post-market Surveillance: Complaints handling and recalls.
Stay compliant and build trust with ISO certification!
Reach out to our team at [email protected].
Benefits of ISO Certifications for Skincare Product Manufacturing
There’s no overstating the advantages of ISO certification in today’s competitive beauty landscape:
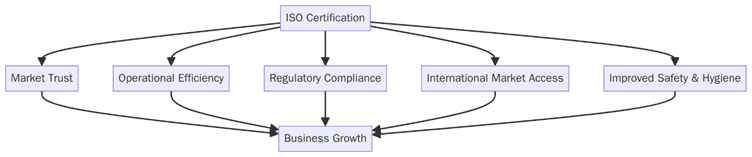
Product Safety:
ISO 22716 ensures controlled environments for production, minimizing contamination risks.
Market Access:
ISO standards open doors to international markets by meeting global regulatory expectations.
Brand Trust:
Consumers and stakeholders gain confidence in a brand that is ISO-certified.
Efficiency:
Standardization leads to streamlined processes, reduced waste, and optimized resource use.
Employee Safety:
ISO 45001 ensures safe workplaces, boosting morale and reducing downtime.
Sustainability:
ISO 14001 showcases a brand’s commitment to eco-conscious practices.
The global skincare market is expected to reach USD 500 billion by 2030, driven by trends such as personalization, clean beauty, and tech-integrated skincare routines. Regulatory authorities are also tightening compliance expectations, making third-party certifications more relevant than ever.
A recent survey from Statista indicates that 78% of skincare consumers trust brands more when they hold ISO or GMP certifications. Furthermore, with regulatory convergence between the EU, ASEAN, and GCC regions, ISO 22716 is increasingly being mandated for import/export clearance in these territories.
If you're ready to take your skincare manufacturing to the next level of compliance, quality, and global competitiveness, Pacific Certifications is here to assist. We specialize in certification audits that validate your processes against ISO benchmarks!
Pacific Certifications is accredited by ABIS, in case you need support with ISO certification for your Skincare Product Manufacturing business, please contact us at [email protected] or +91-8595603096.
Read More: Pacific Blogs

