ISO 9001 vs ISO 13485: Understand the Differences

Introduction
Selecting between ISO 9001 vs ISO 13485 can significantly influence how organizations manage quality, risk and compliance across their operations. ISO 9001 establishes a universal framework for quality management that applies to any industry, while ISO 13485 is specifically tailored for medical devices, incorporating rigorous risk management, design verification and post-market processes. Understanding the difference between the two helps organizations choose the right system, meet regulatory expectations and enhance market credibility.
Begin your ISO certification journey with Pacific Certifications and align your quality systems to the right global standard.
Quick summary
ISO 9001 focuses on general quality management, emphasizing customer satisfaction and continuous improvement across industries. ISO 13485, on the other hand, is designed for medical device organizations, integrating product safety, lifecycle risk control and regulatory conformity. Many medical manufacturers hold both certifications to maintain alignment with global markets and supply chain expectations.
Why the difference matters for medical device supply chains?
Medical devices are highly regulated because of their impact on patient safety. ISO 13485 builds on ISO 9001 principles but adds strict requirements for risk management, design validation and traceability that align with medical device laws and approvals. ISO 9001 remains suitable for general manufacturers and suppliers outside the regulated scope. Selecting the correct standard ensures that processes, documentation and audits align with both operational needs and regulatory expectations.
ISO 9001 provides consistency and customer confidence; ISO 13485 introduces the safeguards and documentation rigor that medical devices require.
Begin your ISO certification journey with Pacific Certifications and align your quality systems to the right global standard.
ISO 9001 vs ISO 13485 - key differences at a glance
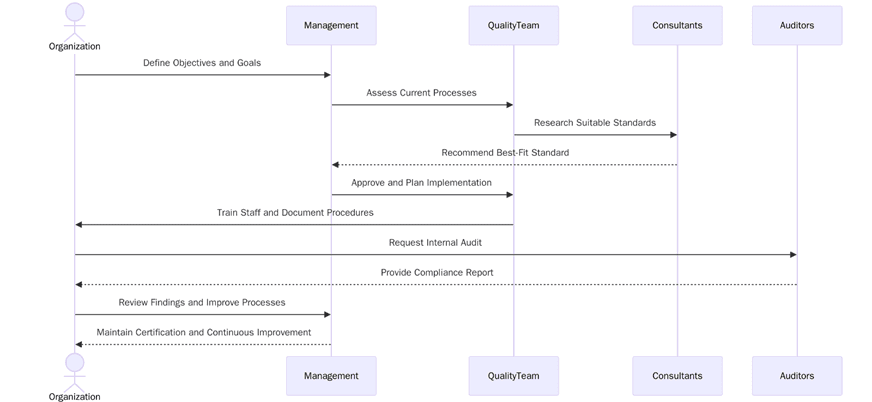
What are the requirements to choose and implement the right standard between ISO 9001 vs ISO 13485?
Implementing the right quality management system starts with understanding scope, regulatory obligations and stakeholder needs. Below are the key requirements:
Define the scope, including all products, services and related processes.
Identify applicable regulations and quality expectations in each target market.
Establish quality policies and objectives that reflect customer and safety priorities.
Develop documented procedures for document control, supplier management, training and corrective actions.
Implement process controls to manage design, production and traceability.
Conduct risk assessments to identify potential failures and implement mitigation actions.
Maintain evidence through records, reports and performance monitoring.
Conduct internal audits and management reviews to ensure ongoing improvement.
Provide employee training to strengthen quality awareness and competence.
Tip:If your organization supplies critical components to a medical device manufacturer, adopting ISO 13485 can give you a competitive edge and make you a preferred supplier for global buyers.
How to prepare for ISO 9001 vs ISO 13485 certification?
Preparation involves documenting your processes, identifying gaps and ensuring leadership involvement. Below are the key preparation steps:
Perform a gap analysis comparing your current processes against ISO clauses.
Update documentation and procedures based on your chosen standard.
Train employees to understand quality objectives and audit readiness.
Qualify and evaluate suppliers through set criteria and measurable KPIs.
Conduct internal audits and management reviews before the external audit.
Address nonconformities promptly and record corrective actions.
Prepare evidence packages that demonstrate control across all processes.
Certification audit for ISO 9001 vs ISO 13485
Stage 1 audit: Review of scope, documented processes and preparedness.
Stage 2 audit: On-site or remote evaluation of implementation and effectiveness.
Nonconformities: Must be resolved with evidence of corrective actions.
Management review: Ensures leadership oversight and system improvement.
Final certification: Issued upon verification of compliance.
Surveillance audits: Conducted annually to confirm ongoing performance.
Recertification audits: Occur every three years to renew certification.
What are the benefits of picking the right standard Between ISO 9001 vs ISO 13485?
ISO 9001 helps organizations maintain consistent product and service quality, improving customer satisfaction. ISO 13485, however, enables compliance with international medical device regulations while ensuring patient safety and risk control. Below are the key benefits:
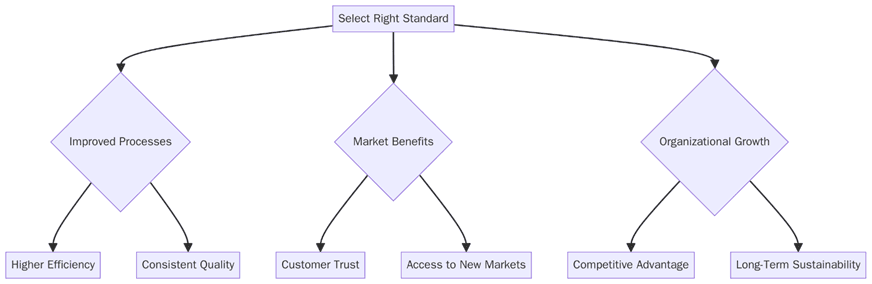
Greater buyer confidence and acceptance in both regulated and non-regulated markets.
Reduced audit findings and improved traceability of critical components.
Stronger supplier relationships through clear standards and consistent oversight.
Lower risk of recalls due to structured risk management and validation.
Enhanced reputation and faster product approvals in global markets.
Recent Trends
Recent data shows that organizations adopting ISO 13485 experience measurable performance gains. Companies using the standard report a 30% reduction in product recalls and significantly faster approval timelines for new devices compared to uncertified peers. Manufacturers are increasingly aligning their systems with ISO 13485 to comply with both EU and U.S. regulatory expectations. Smaller suppliers that previously relied on ISO 9001 are transitioning toward ISO 13485 to meet buyer requirements and strengthen quality assurance. Dual certification is becoming common among global organizations that serve both medical and non-medical markets.
By 2030, ISO 13485 is expected to become a universal benchmark for all medical device-related organizations worldwide. Companies certified under ISO 13485 are projected to experience 20–30% faster product approval timelines and a significant reduction in compliance costs. ISO 9001 will continue evolving as the baseline for non-medical sectors, ensuring organizations maintain global credibility, while dual-certified firms will dominate high-value international supply chains.
Contact us
Pacific Certifications provides accredited audit and certification services for ISO 9001 and ISO 13485. Our certification process ensures that your systems align with international quality expectations and support long-term growth.
Request your ISO audit plan and fee estimate, we will help you map Stage 1 and Stage 2 timelines and evidence requirements for your organization. Contact us at [email protected] or visit www.pacificcert.com
Training and courses
Pacific Certifications provides accredited training programs for ISO 9001 and ISO 13485 to help professionals understand, implement, and audit quality management systems effectively:
· Lead Auditor Training: Designed for professionals responsible for conducting internal and external audits of quality systems within manufacturing and medical device environments.
· Lead Implementer Training: Intended for quality and regulatory personnel involved in establishing or improving management systems based on ISO 9001 or ISO 13485.
Contact [email protected] to schedule your ISO 9001 or ISO 13485 training program and strengthen your organization’s quality and compliance expertise.
Contact Us
Contact Pacific Certifications to begin your certification journey today!
Author: Alina Ansari
If you need support with ISO 9001 or ISO 13485 certification, contact us at [email protected].
Read More at: Blogs by Pacific Certifications

