ISO Certifications for Medical and Scientific Equipment Wholesaling Businesses, Requirements and Benefits

ISO Certifications for Medical and Scientific Equipment Wholesaling
Medical and scientific equipment wholesaling plays a critical role in the healthcare and scientific research sectors. Wholesalers ensure the availability of high-quality and reliable equipment to healthcare providers, laboratories and other industries that rely on advanced technology for patient care and research purposes.
Given the importance of these sectors adopting international standards is vital for maintaining trust and safety across the supply chain.
ISO certifications provide a framework for ensuring that medical and scientific equipment wholesalers meet international standards of quality & safety. These standards are designed to help organizations maintain consistent product quality and to comply with legal and regulatory.
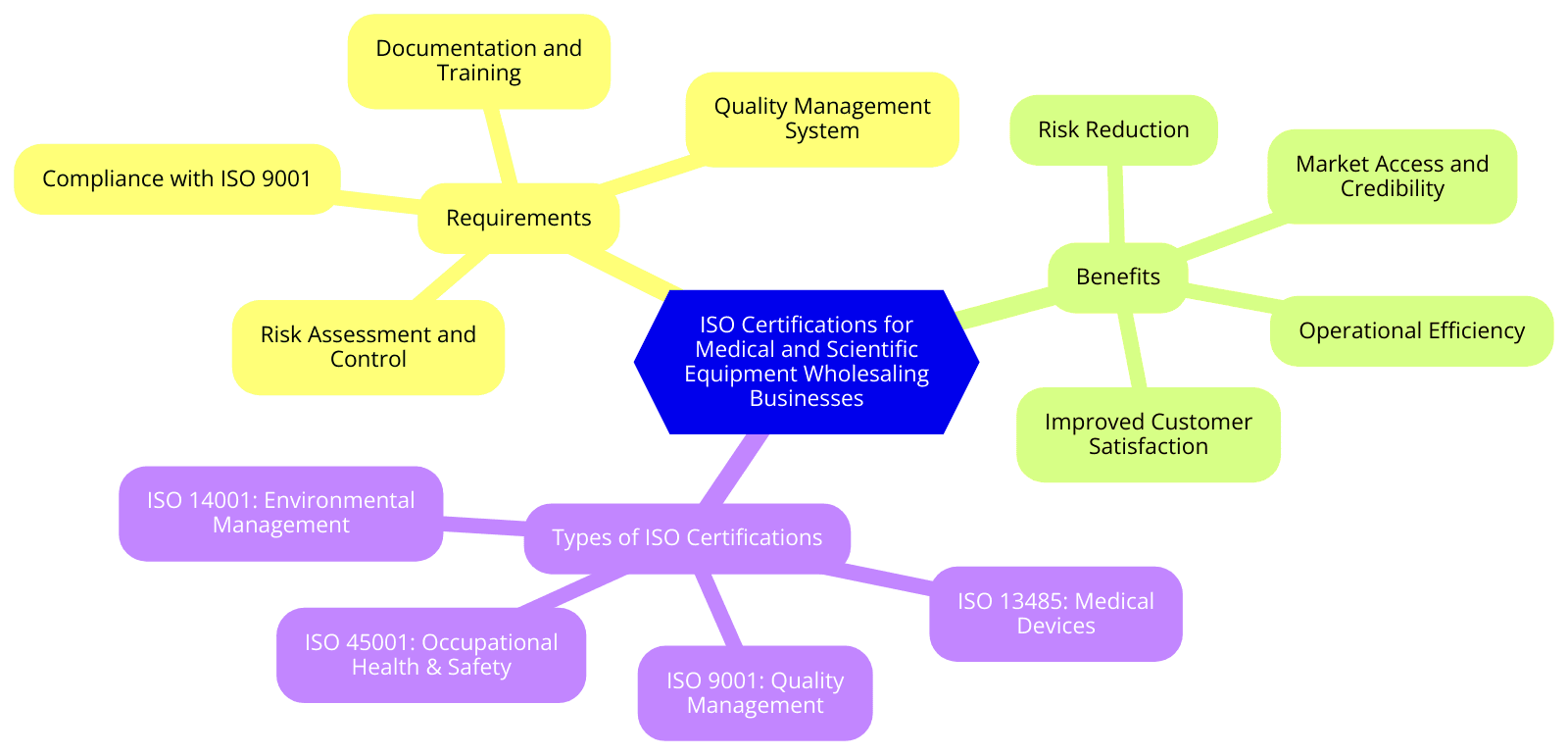
As the healthcare and scientific industries continue to grow and innovate, ISO certifications have become increasingly critical for equipment wholesalers looking increase their credibility.
If you're seeking ISO certification for your Medical and Scientific Equipment Wholesaling business, reach out to us at [email protected] or call +91-8595603096.
Applicable ISO Standards for Medical and Scientific Equipment Wholesaling
ISO standards cover a broad range of quality management and safety criteria that are applicable to the medical and scientific equipment wholesaling industry. Below are some key ISO standards relevant to this sector:
ISO 9001:2015 - Quality Management Systems (QMS)
ISO 9001 sets out the criteria for a quality management system. For medical and scientific equipment wholesalers, this certification ensures that the products and services meet customer and regulatory requirements consistently, ensuring quality control throughout the supply chain.
ISO 13485:2016 - Medical Devices: Quality Management Systems
Specifically tailored to the medical devices industry, ISO 13485 focuses on risk management, traceability, and regulatory compliance in the manufacturing and distribution of medical devices.
ISO 14001:2015 - Environmental Management Systems
ISO 14001 helps organizations manage their environmental responsibilities systematically. For medical and scientific equipment wholesalers, this certification demonstrates a commitment to minimizing environmental impact.
ISO 45001:2018 - Occupational Health and Safety Management Systems
Health and safety in the workplace are essential, especially in sectors handling sensitive and high-risk equipment. ISO 45001 helps wholesalers maintain a safe working environment and reduce risks related to occupational health and safety.
ISO 27001:2022 - Information Security Management Systems
ISO 27001 ensures that sensitive data, including customer details and proprietary product information, is protected from unauthorized access and breaches.
ISO 37001:2016 - Anti-Bribery Management Systems
ISO 37001 helps organizations in the medical and scientific equipment wholesale industry to implement policies and controls to detect, prevent, and respond to bribery. Adherence to this standard promotes integrity and ethical business practices.
ISO 22301:2019 - Business Continuity Management Systems
Ensuring the uninterrupted availability of medical and scientific equipment is critical, especially in times of crisis or disaster. ISO 22301 supports wholesalers in maintaining business continuity and recovering quickly from disruptions.
Click here to find out more applicable standards to your industry
Ready to elevate your Medical and Scientific Equipment Wholesaling operations with ISO certification? Contact us at [email protected] or give us a call at +91-8595603096.
How We Can Help?
At Pacific Certifications, we specialize in helping medical and scientific equipment wholesalers achieve and maintain ISO certifications. As a certification body, we provide independent, third-party audits to verify compliance with ISO standards. Our role is limited to conducting audits and issuing certifications.
We understand the critical role ISO certifications play in maintaining quality and compliance in the medical sector. Through our thorough and impartial auditing process, we ensure that organizations meet the requirements of international standards.
Our expert auditors have extensive experience in the healthcare and scientific industries, ensuring that audits are carried out to the highest standards, with a focus on accuracy and efficiency.
For expert guidance in ISO certification for Medical and Scientific Equipment Wholesaling, connect with us at [email protected] or call +91-8595603096 today.
Requirements of ISO Certifications for Medical and Scientific Equipment Wholesaling
Each ISO standard has its own set of requirements that medical and scientific equipment wholesalers must meet to obtain certification. Below is an overview of the mandatory requirements for the most relevant ISO standards:
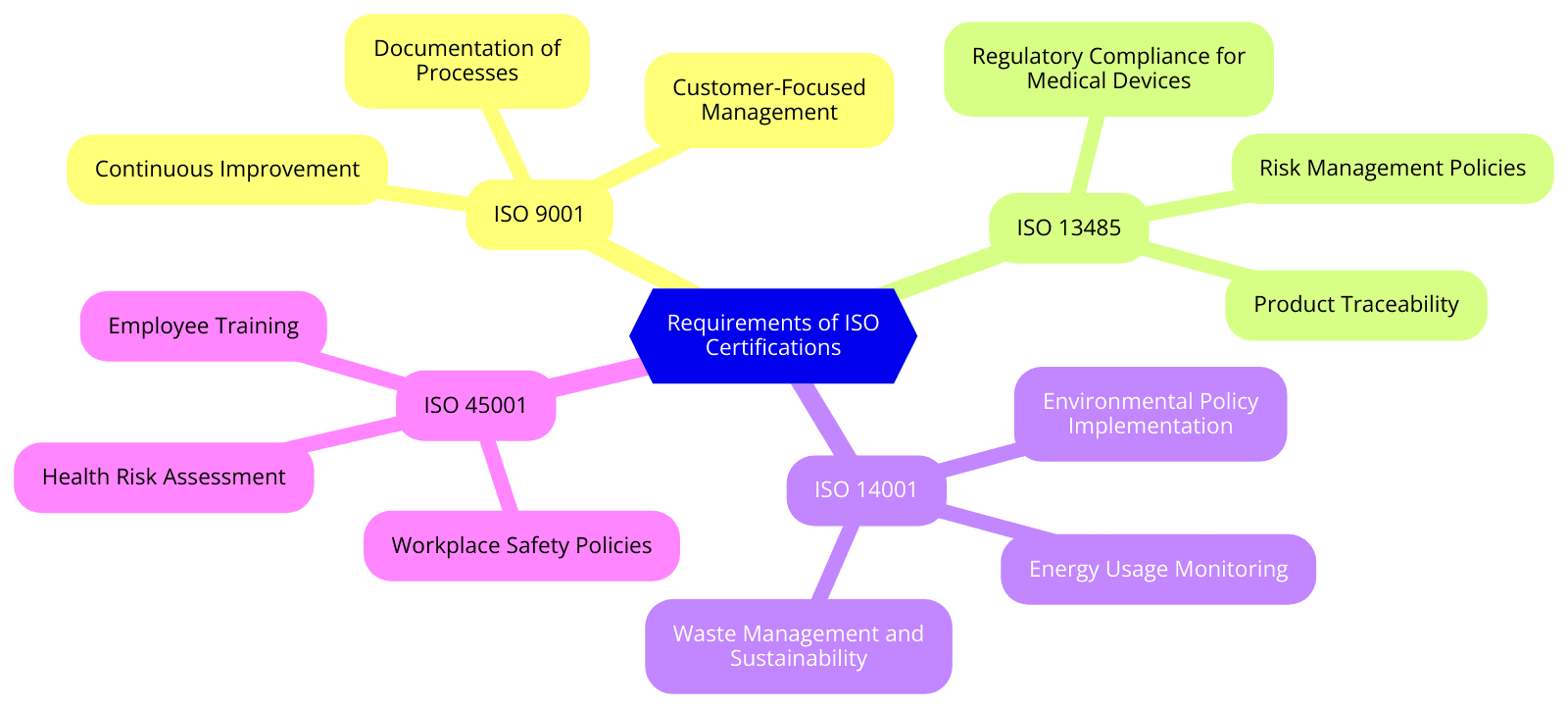
ISO 9001:2015 requirements:
- Establish and document a quality management system that includes policies, processes, and procedures to ensure consistent product quality.
- Maintain records that demonstrate compliance with customer and regulatory requirements.
- Implement a system for continuous improvement, including regular internal audits and corrective actions.
ISO 13485:2016 requirements:
- Develop a documented quality management system tailored to the medical device industry.
- Implement risk management practices to address potential hazards related to medical equipment.
- Ensure full traceability of medical devices throughout the supply chain.
ISO 14001:2015 requirements:
- Identify and assess the environmental impact of business activities and implement measures to reduce environmental harm.
- Establish an environmental management system (EMS) with clear objectives and targets.
- Monitor and review environmental performance regularly.
ISO 45001:2018 requirements:
- Develop a robust health and safety management system.
- Conduct regular risk assessments to identify and mitigate workplace hazards.
- Ensure compliance with occupational health and safety regulations.
ISO 27001:2022 requirements:
- Implement an information security management system (ISMS) to protect sensitive information.
- Conduct risk assessments to identify potential security threats and vulnerabilities.
- Implement security controls and procedures to mitigate risks.
Ensure compliance and quality in your Wholesaling business with ISO certification. Contact us now at [email protected] or call +91-8595603096.
Benefits of ISO Certifications for Medical and Scientific Equipment Wholesaling
Here are the specific benefits of the common ISO standards:
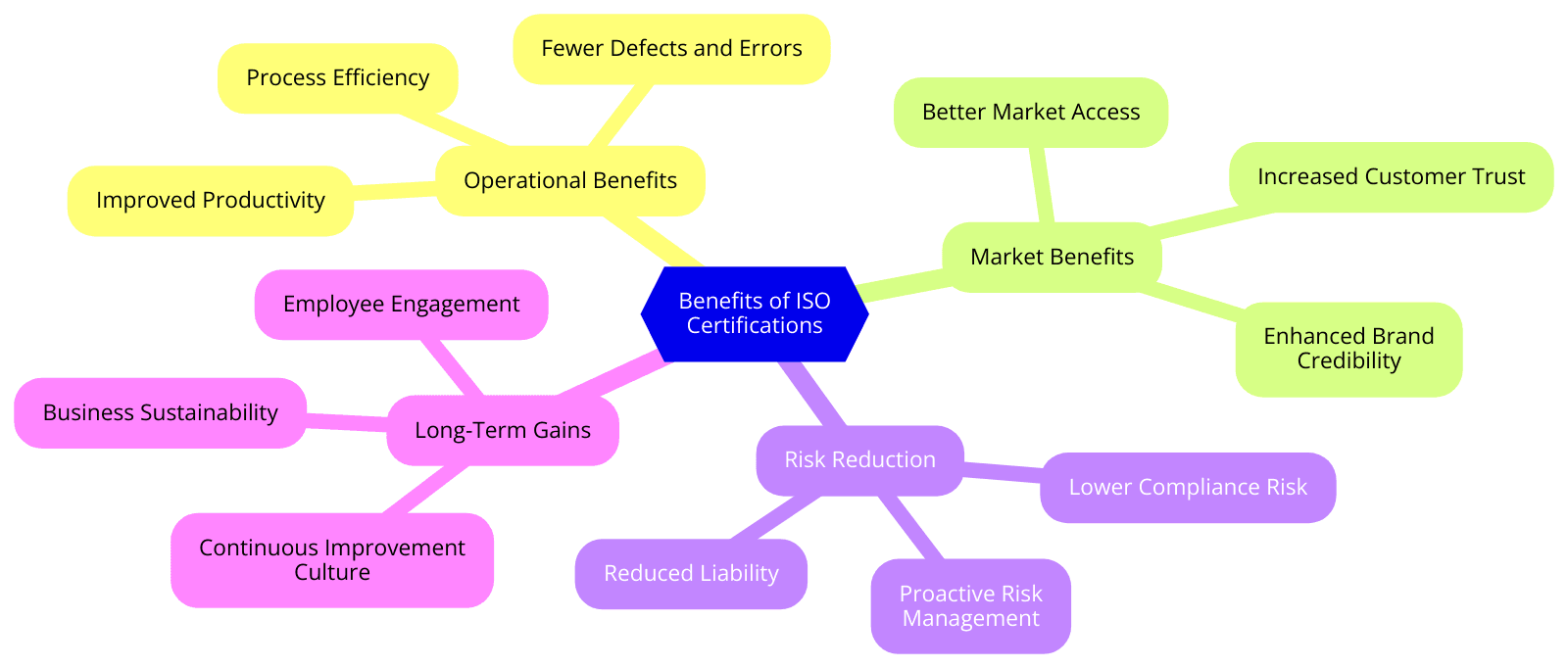
- ISO 9001 ensures that businesses meet customer needs consistently, which enhances customer satisfaction and loyalty.
- Compliance with ISO 9001 often aligns with regulatory requirements, helping organizations meet legal obligations.
- ISO 13485 emphasizes risk management and quality control in medical device production, reducing the likelihood of defects and recalls.
- ISO 13485 helps companies comply with regulatory requirements in medical device production, facilitating access to international markets.
- ISO 14001 helps organizations systematically reduce their environmental footprint by managing waste, emissions, and energy use.
- ISO 45001 helps organizations create safer workplaces by identifying and mitigating health and safety risks.
- ISO 27001 protects sensitive data from cyber threats by implementing robust security measures and controls.
- ISO 37001 helps organizations implement policies to detect and prevent bribery, fostering ethical business practices.
- ISO 22301 helps organizations prepare for and recover from disruptions, ensuring business continuity during crises.
This is how ISO standards support medical equipment wholesalers in achieving operational excellence and maintaining compliance. Interested in ISO certification for your Medical Equipment Wholesaling company? Get in touch with us at [email protected] or call +91-8595603096.
Market Trends
The global market for medical and scientific equipment is growing rapidly, driven by technological advancements and the increasing demand for high-quality healthcare. The global medical equipment wholesaling industry is expected to reach a value of over $600 billion by the end of 2024.
Innovations in telemedicine, remote diagnostics, and AI-powered medical devices are driving the demand for high-tech equipment. Additionally, the COVID-19 pandemic has accelerated the adoption of new medical technologies and increased the focus on supply chain resilience.
In near future, wholesalers will face increased pressure to ensure the availability of advanced equipment and comply with stringent regulatory standards. ISO certifications will play a crucial role in helping businesses meet these challenges and remain competitive.
Start your journey toward ISO certification in Medical and Scientific Equipment Wholesaling today by contacting us at [email protected] or +91-8595603096.
Pacific Certifications is accredited by ABIS, in case you need support with ISO certification for your Medical and Scientific Equipment Wholesaling business, please contact us at [email protected] or +91-8595603096.
Ready to get ISO certified?
Contact Pacific Certifications to begin your certification journey today!
Suggested Certifications –

