ISO 9001 vs ISO 22000: Comparing Quality and Food Safety Systems

Introduction
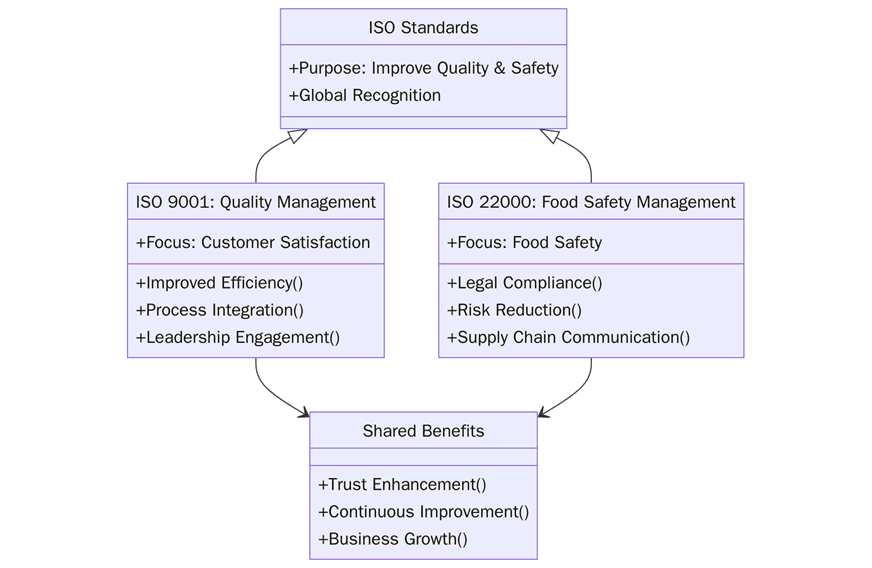
Quality and food safety share many building blocks but they solve different problems. ISO 9001 sets a Quality Management System (QMS) that works for any industry and focuses on meeting customer needs through controlled processes and continual improvement. ISO 22000 sets a Food Safety Management System (FSMS) that combines ISO management practices with HACCP so food hazards are identified, controlled, and traced across the supply chain. Knowing where they align and where they differ helps an organization pick the right scope, avoid rework, and satisfy buyer and regulator expectations in one pass.
Book a 20-minute scoping call with Pacific Certifications to map ISO 9001 vs ISO 22000 for your operation.
Quick summary
ISO 9001 is the universal template for managing quality in products and services. ISO 22000 is tailored for food chains and builds hazard analysis, critical control points, prerequisite programs, and traceability into a single system. Many food businesses use both: ISO 9001 to keep processes stable and ISO 22000 to keep food safe from farm to fork.
Why the difference matters for food businesses?
Food brands live and die by trust. An ISO 9001 system stabilizes planning, production, and customer processes so defects and complaints fall. An ISO 22000 system goes further by hardwiring hazard analysis, CCP control, supplier hygiene, allergen control, and recall readiness. Using the right standard or an integrated set - lowers risk, shortens audits, and makes acceptance by retailers and export markets far smoother.
“While ISO 9001 establishes the foundation for organizational consistency, efficiency, and customer satisfaction across all sectors, ISO 22000 builds on these principles by embedding food-specific risk controls, traceability, and regulatory alignment. Together, they form a continuum where quality assurance and food safety converge to protect consumers, strengthen brand credibility, and enable sustainable global trade.”
ISO 9001 vs ISO 22000 - side-by-side comparison
What are the requirements for ISO 9001 and ISO 22000?
Before selecting one or both, set scope, map risks, and lock in evidence that proves control day to day. Below are the key requirements for a clean rollout that holds up in audits and buyer reviews:
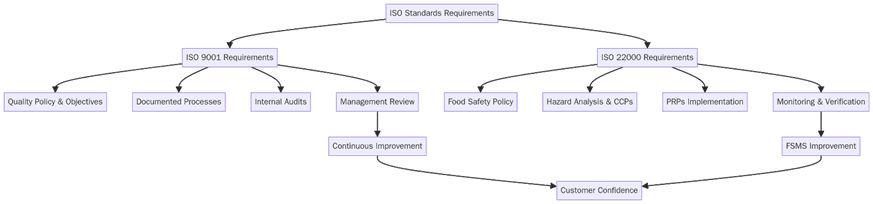
Define organizational boundaries and processes in scope, including outsourced steps.
Set policies and measurable objectives for quality or food safety that connect to customer and legal needs.
Build documented procedures and records for planning, operations, purchasing, production, and service.
Run risk assessments: process risks for ISO 9001, food hazards and CCPs for ISO 22000.
Establish monitoring, measurement, and verification methods with clear acceptance criteria.
Train staff on roles, hygiene, CCP monitoring, verification, and incident reporting.
Control suppliers with approval criteria, PRP expectations, and incoming checks.
Keep traceability, nonconformity handling, and recall or complaint workflows ready to use.
Perform internal audits and management reviews; close findings with effective actions.
Stage evidence packs for Stage 1 and Stage 2 certification audits.
Tip:If you already run HACCP, migrate your hazard analysis and CCP documentation into the ISO 22000 structure first, then layer ISO 9001 clauses for purchasing, design, service, and improvement to create a single integrated system.
How to prepare for certification?
A smooth audit depends on clear scope, tidy records, and people who know their roles. Use these steps to remove surprises:
Run a gap analysis against ISO 9001 and ISO 22000 clauses to see what already fits.
Align procedures, forms, and logs so the same record can serve both quality and food safety needs.
Map PRPs site by site: cleaning, sanitation, allergen control, water quality, pest control, glass and brittle plastics.
Validate CCP critical limits and verification methods; document rationale.
Train operators and supervisors on monitoring, escalation, and evidence capture.
Pilot a mock recall and a mock customer complaint to test traceability and response speed.
Hold an internal audit and a management review; fix gaps before Stage 1.
Certification audit
Stage 1 audit: Readiness review of scope, policies, risk methods, HACCP study, and records.
Stage 2 audit: Evaluation of implementation in production, warehouse, transport, and supplier control.
Nonconformities: Must be corrected with proof and verified for closure.
Management review: Confirms leadership oversight and resource support.
Final certification: Issued after gaps close and evidence meets requirements.
Surveillance audits: Conducted annually to verify ongoing control.
Recertification audits: Every three years to renew the certificate.
What are the benefits of ISO 9001 and ISO 22000?
A well-built QMS and FSMS improve daily control, speed audits, and protect brand value. Below are the key benefits of using ISO 9001, ISO 22000, or both in an integrated system:
Stronger process reliability with clear SOPs, role clarity, and training that cuts rework.
Fewer complaints through better specifications, in-process checks, and corrective actions.
Lower food safety risk with hazard analysis, CCP control, and verified PRPs.
Faster buyer approval because records match retailer and import expectations.
Better supplier performance with defined criteria, audits, SLAs, and KPIs.
Sharper traceability that shortens recall time and limits affected lots.
Leaner audits since one system covers quality, hygiene, and legal needs.
Reduced waste and losses by preventing defects, cross-contamination, and mislabeling.
Higher staff ownership from simple visual controls and daily checks.
Improved market access for exports and private label programs.
Market Trends
Many food organizations report double-digit drops in consumer complaints after ISO rollout, recall readiness that moves from days to hours, and measurable gains in right-first-time output once CCP monitoring becomes routine. Over multi-year cycles, integrated systems tend to lower incident frequency, shorten audit closure time, and raise customer retention in private label and contract manufacturing channels.
Food businesses are consolidating ISO 9001 and ISO 22000 into one integrated framework so the same audits, KPIs, and reviews serve both quality and safety. Retailers ask for deeper supplier visibility, so plants are adding digital traceability, allergen mapping, and camera-assisted CCP checks. Multi-site groups are harmonizing PRPs and recall playbooks to speed cross-site rollouts and reduce variation. By 2030, expect wider use of real-time sensors and line-side analytics for CCP monitoring, automated trend alerts for hygiene nonconformities, and faster buyer portal uploads for audits and KPIs. Integrated QMS-FSMS platforms will dominate new builds, with suppliers measured on response time to incidents, audit closure days, and proof of allergen and foreign-body controls during seasonal peaks.
Training and courses
Pacific Certifications provides accredited training programs tailored to organizations comparing or integrating ISO 9001 and ISO 22000. These programs build audit-ready competence across quality management and food safety management, including HACCP, CCP monitoring, and supplier controls.
· Lead Auditor Training: For professionals auditing ISO 9001 and ISO 22000 systems in manufacturing, processing, distribution, and catering.
· Lead Implementer Training: For personnel establishing or improving integrated QMS and FSMS.
Contact [email protected] to schedule ISO 9001 and ISO 22000 training or awareness sessions and strengthen your team’s capability across quality and food safety.
How Pacific Certifications can help?
Pacific Certifications provides accredited ISO certification services to organizations across industries. Our audits help institutions improve system performance, maintain compliance, and strengthen credibility with clients and regulators. We do not offer consultancy, we focus purely on impartial audit and certification.
Request your ISO audit plan and fee estimate. We will help you map Stage-1/Stage-2 timelines and evidence requirements for your organization. Contact us at [email protected] or visit www.pacificcert.com.
Contact Us
Contact Pacific Certifications to begin your certification journey today!
Author: Alina Ansari
Read more: Pacific Blogs

