ISO 13485: QMS Requirements of Medical Devices and Risk Management

Introduction
The medical device industry operates in one of the most sensitive and highly regulated environments. Every component, process and decision can directly affect patient safety and clinical outcomes, making a structured Quality Management System (QMS) essential. ISO 13485 provides the global foundation for designing, manufacturing and distributing medical devices with consistency and safety. It reinforces rigorous process control, risk-based thinking, product traceability and strong documentation practices across the device lifecycle.
As global regulatory expectations increase and health systems demand greater transparency from suppliers, ISO 13485 certification has become a core requirement for manufacturers, component makers, sterilization providers, distributors and service providers. Implementing this standard helps institutions detect risks early, strengthen design controls and establish clear quality responsibilities across teams. In an industry where reliability is non-negotiable, ISO 13485 forms the backbone of trust, compliance and long-term credibility.
Speak with Pacific Certifications to understand how an accredited ISO 13485 audit can strengthen your medical device quality framework.
Quick summary
ISO 13485 sets out the requirements for a QMS tailored for medical devices and related services. It prioritizes risk management, design control, product traceability, documentation integrity and process validation. Institutions use ISO 13485 to ensure safe device performance, meet global regulatory expectations and maintain strong supplier and post-market controls. A well-implemented system reduces defects, strengthens patient safety and positions manufacturers for long-term growth.
Why ISO 13485 and risk management matter today?
Medical devices play a critical role in diagnosis, treatment and patient care. Even small process variations can lead to failures, safety issues, or regulatory delays. ISO 13485 offers a structured system that ensures consistency, accuracy and accountability in device manufacturing. Risk management becomes central—institutions must analyze hazards, evaluate potential failures, track corrective actions and maintain safety data throughout the device lifecycle.
With stronger risk-based thinking, device manufacturers are better prepared for regulatory audits, supplier assessments and global market access requirements.
“ISO 13485 creates a common language for quality and safety, ensuring every stage of the medical device journey is controlled, measured and aligned with clinical expectations.”
Table: ISO 13485 - Core Elements and Risk Management Link
Clause Area | Focus | Risk Management Impact |
QMS Documentation | Policies, procedures, traceability | Clear evidence trail supports risk control |
Management Responsibility | Leadership involvement | Stronger governance for risk-based decisions |
Resource Management | Competence, equipment, environment | Prevents errors from inadequate conditions |
Product Realization | Design to distribution | Full lifecycle risk evaluation |
Measurement & Improvement | CAPA, audits, monitoring | Reduces defects and corrective actions |
What are the requirements of ISO 13485?
ISO 13485 requires institutions to develop a QMS that ensures device safety, consistency and compliance throughout all stages of production and servicing. Before meeting these steps, organizations must first understand the full lifecycle of their products and how risks influence design, development and performance.
Below are the key requirements:
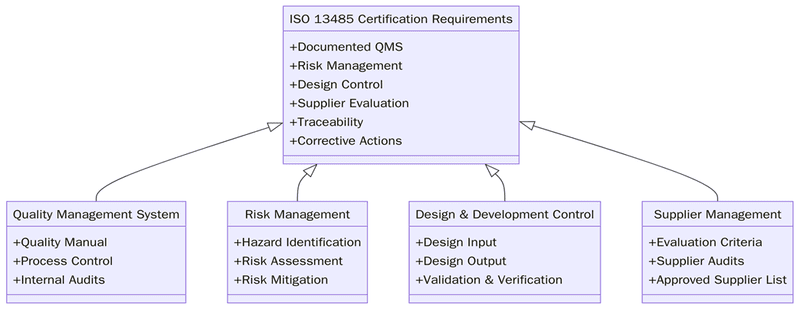
- Establish a documented QMS with defined processes and controls.
- Create a quality manual, procedures and structured records.
- Implement risk management in accordance with device lifecycle needs.
- Control design and development stages through documented design files.
- Validate production and sterilization processes.
- Maintain traceability systems for components and finished devices.
- Establish supplier controls and evaluation mechanisms.
- Ensure equipment calibration and maintenance.
- Implement complaint handling, vigilance and recall procedures.
- Conduct internal audits and management reviews.
- Maintain CAPA processes to address nonconformities.
- Ensure continual improvement is supported by documented evidence.
Tip:Maintain a Design History File (DHF) and Device Master Record (DMR) with up-to-date risk documentation for faster audit readiness.
How to prepare for ISO 13485 certification?
Preparing for ISO 13485 means structuring documentation, reviewing risks and ensuring evidence is complete and traceable. Institutions benefit from establishing dedicated quality roles and conducting internal assessments before certification.
1. Conduct a gap assessment against ISO 13485 requirements.
2. Update QMS documentation and ensure traceability is consistent.
3. Review design control requirements and validate all processes.
4. Strengthen supplier audits and component verification.
5. Confirm calibration and environmental controls are functioning.
6. Train employees on quality, documentation and safety practices.
7. Conduct internal audits and address corrective actions.
8. Hold a management review meeting before external audit.
Certification audit
Stage 1 audit: Reviews documentation, design files, risk management records and QMS policies.
Stage 2 audit: Confirms implementation across production, testing and distribution stages.
Nonconformities: Must be corrected with documented evidence.
Management review: Shows leadership oversight and regulatory alignment.
Final certification: Granted when compliance is verified.
Surveillance audits: Conducted annually to confirm continued control.
Recertification audits: Required every three years to maintain certification status.
What are the benefits of ISO 13485?
Organizations implementing ISO 13485 achieve stronger quality consistency, reduced risk exposure and better market recognition. Before these benefits are realized, institutions often experience improved documentation clarity and stronger communication between departments.
Below are the key benefits:
- Improved device safety through structured QMS controls
- Better documentation integrity and traceability
- Fewer defects and reduced rework through validated processes
- Stronger supplier evaluations and quality oversight
- Easier regulatory approvals due to clear design and risk records
- Increased reliability across production and servicing activities
- Better customer and healthcare provider confidence
- KPIs: defect rate, complaint resolution time, audit closure speed
- SLAs: CAPA response time, supplier approval turnaround, design review intervals
Market Trends
Medical device manufacturers are increasingly adopting digital QMS platforms to improve traceability, automate documentation and support real-time monitoring. There is rising interest in merging ISO 13485 with ISO 14971 for comprehensive risk management. Organizations are also moving toward integrated compliance systems, using automated CAPA workflows, digital signatures and cloud-based document control systems.
In the coming years, quality management in medical devices will rely heavily on predictive analytics, AI-enabled risk modelling and automated traceability systems. Institutions with strong ISO 13485 frameworks will see faster approvals, greater supply chain acceptance and stronger global recognition. As regulatory expectations increase, ISO 13485 will remain essential for device safety and market access.
Training and courses
Pacific Certifications offers accredited training for ISO 13485:
- Lead Auditor Training: For individuals assessing QMS effectiveness, traceability and risk-based controls.
- Lead Implementer Training: For those responsible for establishing or improving medical device quality systems.
To schedule an ISO 13485 training session, contact [email protected].
How Pacific Certifications can help?
Pacific Certifications provides accredited ISO 13485 certification and audit services for medical device manufacturers, component suppliers and service providers. Our audits verify documentation, traceability, risk management and process control. We issue Certificates of Conformity following impartial evaluations, without providing consultancy.
For an ISO 13485 certification plan or audit roadmap, contact [email protected] or visit www.pacificcert.com.
Ready to get ISO 13485 certified?
Contact Pacific Certifications to begin your certification journey today!
Author: Alina Ansari
Suggested Certifications –
Read more: Pacific Blogs

