ISO 13485: QMS Requirements of Medical Devices and Its Importance

Introduction
The medical device industry is one of the most highly scrutinized industries where patient safety, accuracy and traceability denote product success. Every medical device, from surgical instruments to diagnostic systems, must achieve stringent quality expectations to protect safety and reliability. ISO 13485 is the internationally recognized standard for a quality management system (QMS) for medical device manufacturers, supplier and service providers. This standard establishes controls for design, production, installation, and servicing, which provides a systematic framework to mitigate risk while assuring the quality of products and services provided.
As global regulations tighten, ISO 13485 certification has aligned itself as a baseline requirement for entry into markets in the United States, European Union and Asia-Pacific. Certification signifies that an organization maintains compliance with internationally accepted quality and regulatory practices such as the EU MDR (Medical Device Regulation) and US FDA QSR (Quality System Regulation).
Start your ISO 13485 certification planning with Pacific Certifications, connect with an auditor today to evaluate your organization’s readiness and documentation maturity.
Quick summary
ISO 13485:2016 provides a comprehensive quality management framework tailored specifically to the medical device lifecycle. It focuses on regulatory compliance, risk management, design control, traceability, sterile production and post-market feedback. The standard applies to all organizations involved in any phase of the device supply chain, including component manufacturers, assemblers, distributors, sterilization service providers and calibration laboratories.
By implementing ISO 13485, organizations can reduce product defects, improve reliability and ensure global acceptance of their devices through third-party certification.
Why ISO 13485 is important for medical device manufacturers?
For medical device organizations, product failure can have life-threatening consequences. ISO 13485 establishes a structured system to control every stage of the device lifecycle, from raw materials to post-market surveillance. The standard ensures that quality assurance and regulatory compliance go hand-in-hand, creating traceability across all activities that influence product safety and performance.
In an increasingly globalized healthcare market, ISO 13485 certification also serves as a passport to trade. Many international buyers and regulatory bodies require certification as proof of quality system compliance. This strengthens trust with hospitals, regulators and end-users while reducing the need for repeated audits and technical reviews.
“ISO 13485 brings discipline and transparency to the entire medical device lifecycle, transforming quality assurance into patient protection."
ISO 9001 vs ISO 13485 - Comparison of Key Focus Areas
What are the requirements for ISO 13485 certification?
Achieving ISO 13485 certification requires building a robust, risk-based QMS aligned with medical device regulations. Organizations must demonstrate control over every aspect that impacts product quality and patient safety. Below are the key requirements:
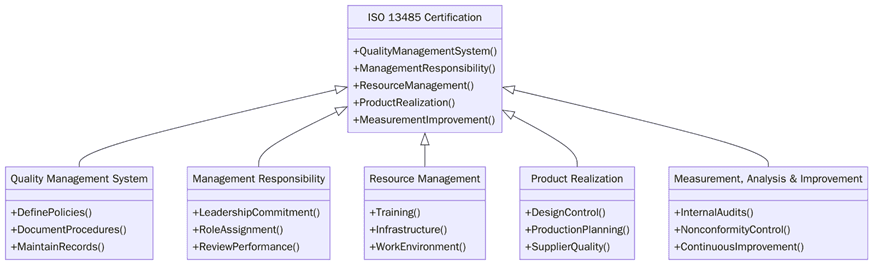
Define the scope of the QMS, covering design, development, manufacturing, storage and servicing.
Establish a quality policy focused on patient safety and regulatory compliance.
Develop documented procedures for design control, change management, validation and verification.
Implement risk management processes aligned with ISO 14971.
Maintain traceability records for materials, components and manufacturing activities.
Create work instructions for production, packaging, labeling and inspection activities.
Establish a supplier evaluation and approval system based on criticality and risk.
Implement nonconformity and corrective action procedures to address product or process deviations.
Conduct internal audits and management reviews to ensure continual compliance.
Maintain a post-market surveillance process for feedback, complaints and field actions.
Tip:Begin implementation with a documentation gap analysis against ISO 13485 clauses, focusing first on high-risk processes such as sterilization, design verification and complaint handling.
How to prepare for ISO 13485 certification?
Preparing for certification requires structured planning, documentation and employee awareness.
Conduct a gap assessment to compare your current QMS with ISO 13485 requirements.
Map process flows for design, production and quality control.
Train employees on regulatory responsibilities and risk management procedures.
Document supplier quality controls and ensure traceability across the supply chain.
Implement internal audits and corrective action plans to close identified gaps.
Hold a management review to verify readiness before scheduling the external audit.
Certification audit
Stage 1 audit: Reviews documentation, quality policies and regulatory controls.
Stage 2 audit: Verifies implementation across manufacturing, inspection, labeling and post-market processes.
Nonconformities: Must be addressed with corrective action and evidence of closure.
Management review: Confirms leadership commitment to regulatory compliance and quality improvement.
Final certification: Awarded once corrective actions are verified.
Surveillance audits: Conducted annually to maintain certification status.
Recertification audits: Conducted every three years to ensure ongoing conformity and continual improvement.
What are the benefits of ISO 13485 certification?
Implementing ISO 13485 delivers measurable benefits across product quality, risk management and global market access. Below are the key advantages:
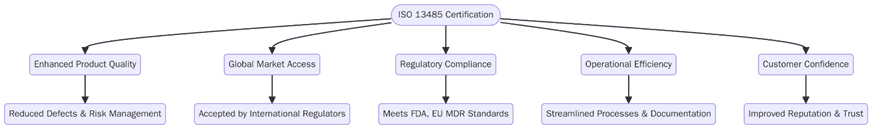
Enhanced product reliability and reduced recall rates.
Stronger compliance with global medical device regulations.
Improved traceability from raw material to end-user delivery.
Consistent risk management integrated with ISO 14971 principles.
Increased confidence among regulators, distributors and healthcare providers.
Streamlined supplier management and quality oversight.
Reduced nonconformities through structured root cause analysis.
Expanded international market access through recognized certification.
Improved audit readiness for MDR, IVDR and FDA inspections.
Better control over documentation, labelling and process validation.
Recent Trends
The medical device sector is witnessing accelerated integration of digital quality management systems, remote auditing and AI-assisted data traceability. In 2025, ISO 13485-certified organizations are leveraging analytics to monitor complaint patterns, device performance and supplier deviations in real time. The rise of connected health devices has also increased focus on integrating ISO 13485 with ISO/IEC 27001 for information security and ISO 22301 for business continuity.
By 2030, ISO 13485 is expected to evolve alongside regulatory frameworks to address digital health devices, AI-assisted diagnostics and additive manufacturing. Manufacturers implementing early digital QMS platforms can expect a 25–30% reduction in documentation errors and 40% faster response times during regulatory inspections. Certification will remain central to demonstrating compliance in the evolving landscape of global healthcare manufacturing.
Training and courses
Pacific Certifications provides accredited training programs for ISO 13485, enabling professionals to build technical and auditing competence for medical device quality management:
· Lead Auditor Training: For professionals responsible for auditing and evaluating ISO 13485-based QMS. It covers audit planning, execution, nonconformity management and reporting aligned with ISO 19011.
· Lead Implementer Training: For personnel responsible for developing and maintaining ISO 13485 systems. It focuses on documentation, risk management integration (ISO 14971), supplier evaluation and post-market surveillance.
To schedule your ISO 13485 training or awareness session, contact [email protected].
How Pacific Certifications can help?
Pacific Certifications provides ISO 13485 certification and audit services for organizations involved in the design, manufacture and distribution of medical devices. Our accredited audits evaluate process control, documentation and regulatory compliance in line with ISO 13485:2016.
We issue Certificates of Conformity after impartial third-party assessments that verify compliance with ISO 13485 and applicable regulatory standards. Pacific Certifications focuses exclusively on auditing and certification, not consulting, ensuring transparency and independence throughout the certification process.
Contact Us
To request your ISO 13485 audit plan and Stage-1/Stage-2 schedule, contact [email protected] or visit www.pacificcert.com.
Read more: Pacific Blogs

