ISO 13485 Certification Guide for Medical Devices and Quality Management

Introduction
The medical device industry demands precision, safety and reliability in every stage of design, production and delivery. ISO 13485 serves as the global benchmark for ensuring that medical device manufacturers meet consistent quality and regulatory standards. It provides a framework for organizations to demonstrate control over the entire product lifecycle, from design and development to distribution and post-market support.
In recent years, ISO 13485 certification has become more than a quality badge; it is a market entry requirement for suppliers, component makers and healthcare device manufacturers worldwide. As regulators tighten expectations and buyers demand certified suppliers, understanding ISO 13485 is crucial for maintaining trust and access to global markets.
Start your ISO 13485 audit readiness discussion with Pacific Certifications, an accredited certification body recognized by ABIS.
Quick summary
ISO 13485 sets requirements for a Quality Management System (QMS) tailored to medical devices and related services. It helps institutions manage risk, meet regulatory obligations and ensure consistent product quality. The standard aligns closely with FDA and EU MDR expectations, supporting traceability, documentation control and product safety throughout the supply chain.
Why ISO 13485 matters in medical manufacturing?
For medical device organizations, ISO 13485 certification validates that products are safe, consistent and compliant with global quality requirements. It reduces regulatory burden by aligning documentation, training and validation processes across jurisdictions.
This standard supports manufacturers, component suppliers and distributors in minimizing defects, improving patient safety and maintaining trust among healthcare professionals and regulatory authorities.
“ISO 13485 creates a single language of quality and safety across the global medical device supply chain — from component design to clinical use.”
Table: ISO 13485 Key Focus Areas
What are the requirements for ISO 13485 certification?
To achieve ISO 13485 certification, organizations must establish processes that ensure product quality and patient safety throughout their operations. Below are the key requirements:
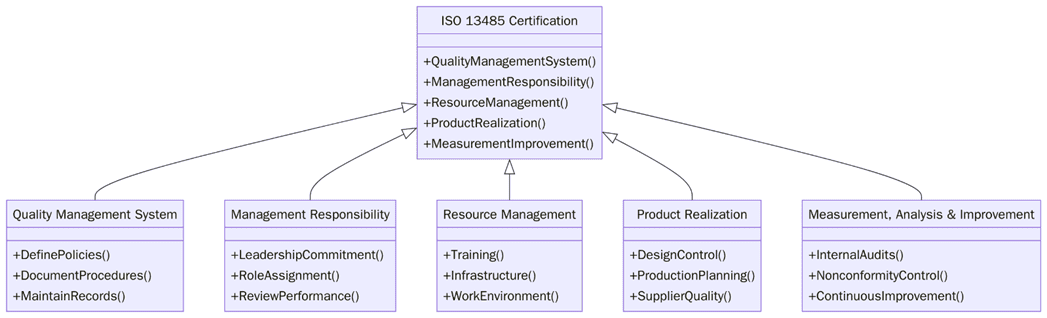
1. Define a QMS covering design, development, production and servicing.
2. Develop documentation such as the quality manual, procedures and records.
3. Conduct risk management for all product stages.
4. Implement supplier evaluation and control mechanisms.
5. Validate all production and sterilization processes.
6. Establish traceability and labelling systems.
7. Maintain complaint handling and recall procedures.
8. Conduct internal audits and management reviews.
9. Train employees on QMS awareness and regulatory updates.
10. Maintain continual improvement and corrective action records.
Tip:Use digital validation tools and electronic document management to simplify evidence tracking and traceability.
How to prepare for ISO 13485 certification?
Preparation involves aligning all product and process documentation with ISO requirements and conducting internal reviews.
1. Perform a gap assessment against ISO 13485:2016 requirements.
2. Identify critical product safety and design validation controls.
3. Create or update the Design History File (DHF) and Device Master Record (DMR).
4. Ensure calibration and maintenance logs are current.
5. Conduct internal audits and resolve nonconformities.
6. Train employees on risk-based thinking and documentation control.
7. Schedule a management review meeting before external audit.
Certification audit
Stage 1 audit: Evaluates documentation, QMS structure and readiness.
Stage 2 audit: Examines implementation across manufacturing, testing and servicing.
Nonconformities: Must be addressed with evidence of correction.
Management review: Confirms leadership oversight and compliance alignment.
Final certification: Issued upon satisfactory completion of corrective actions.
Surveillance audits: Conducted yearly to verify ongoing performance.
Recertification audits: Every three years to renew certification validity.
What are the benefits of ISO 13485 certification?
ISO 13485 certification brings trust, efficiency and compliance assurance. It reduces risk exposure and strengthens competitiveness across global markets. Below are the key benefits:
Improved product quality and patient safety through risk-based controls
Streamlined documentation and traceability across the supply chain
Easier market access through global regulatory alignment
Reduced product recalls and defect rates
Enhanced supplier management and qualification process
Increased customer and regulator confidence
Data-backed decision-making through performance monitoring
KPIs: defect density, recall frequency, audit closure time, supplier compliance rate
SLAs: CAPA response time, complaint resolution time, validation turnaround
Market Trends
The medical device industry is embracing digital QMS tools, AI-driven quality monitoring and paperless validation systems. Manufacturers are integrating ISO 13485 with ISO 14971 (Risk Management) and ISO 9001 to build unified compliance frameworks. With MDR/IVDR tightening globally, ISO 13485 serves as the foundation for maintaining technical documentation and post-market surveillance.
By 2030, ISO 13485-certified organizations will leverage predictive analytics and digital twins to monitor quality performance. Regulatory convergence will make certification an expected prerequisite for market entry. Organizations investing in traceable, AI-assisted QMS will gain faster product approvals and improved safety analytics.
Training and courses
Pacific Certifications provides accredited training programs for ISO 13485:
1. Lead Auditor Training: For professionals responsible for auditing medical device QMS systems and supplier processes.
2. Lead Implementer Training: For individuals overseeing design, production and regulatory compliance integration.
To schedule ISO 13485 training or awareness sessions, contact [email protected].
How Pacific Certifications can help?
Pacific Certifications provides ISO 13485 certification and audit services to medical device manufacturers, component suppliers and distributors. Our independent audits assess system effectiveness, documentation and regulatory alignment.
We issue Certificates of Conformity following impartial assessments, supporting compliance without providing consultancy services.
Contact Us
For your ISO 13485 certification roadmap, contact [email protected] or visit www.pacificcert.com.
Read more: Pacific Blogs

