ISO 13485 Certification for Medical Devices & Components

Introduction
Medical devices play a critical role in modern healthcare, from simple syringes and surgical masks to complex diagnostic equipment and implantable devices. Because they directly affect patient health and safety, regulatory bodies and healthcare providers demand the highest standards of quality and reliability. ISO 13485 certification provides a globally recognized framework for manufacturers and suppliers of medical devices and components to demonstrate that their quality management systems (QMS) meet international expectations for design, production, and post-market monitoring.
With increasingly strict regulations worldwide, including EU MDR, U.S. FDA requirements, and global harmonization efforts, ISO 13485 has become a cornerstone for medical device organizations seeking access to international markets. Certification ensures not only product quality but also patient safety, regulatory compliance, and long-term business growth.
Begin your ISO 13485 certification process with Pacific Certifications to build lasting confidence in your medical devices, components, and manufacturing practices.
Quick summary
ISO 13485 is the international standard for quality management systems specific to medical devices. It ensures safety, effectiveness, and regulatory compliance throughout the device lifecycle, from design and development to manufacturing, distribution, and post-market activities. Certification builds credibility, supports regulatory submissions, and assures healthcare providers and patients of product reliability.
Why ISO 13485 matters for medical devices?
The medical device industry is highly regulated because product failures can have life-threatening consequences. ISO 13485 matters because it integrates quality management with regulatory requirements, ensuring traceability, risk management, and effective monitoring of devices in use. According to Deloitte’s 2024 MedTechreport, companies with ISO 13485 certification were 40% more successful in gaining international market approvals than those without certification.
By adopting ISO 13485, manufacturers and component suppliers gain a structured framework to reduce errors, improve product safety, and satisfy both regulators and buyers. Certification also provides global recognition, which is increasingly vital as healthcare systems demand transparency in supply chains.
ISO 13485 is not only about quality control, it is about safeguarding patient lives by ensuring medical devices are safe, reliable, and effective.
Relevant ISO standards for medical devices
What are the requirements for ISO 13485 certification?
To achieve ISO 13485 certification, organizations must implement a QMS that addresses device safety, regulatory requirements, and lifecycle traceability. Below are the key requirements:
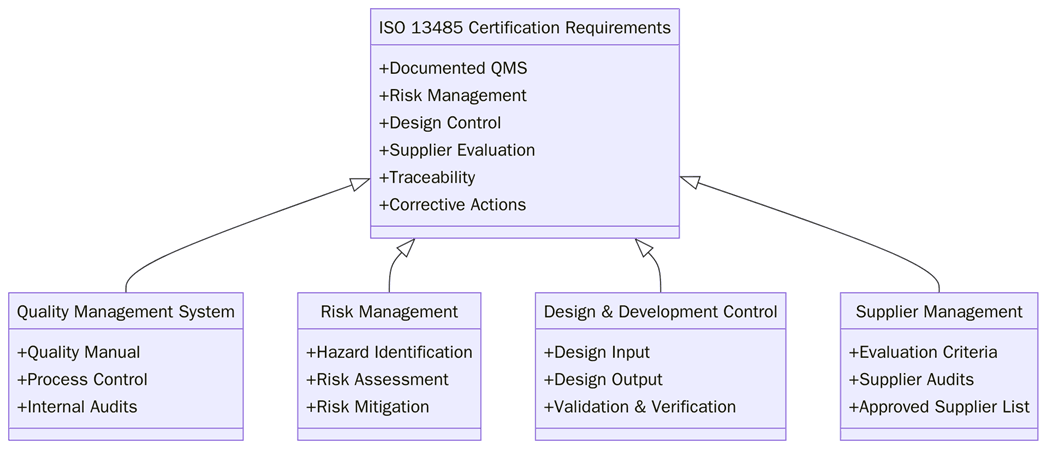
Define scope of the QMS, including devices, components, and services.
Develop documented quality and risk management policies.
Conduct design controls and maintain complete device history files.
Perform risk assessments and ensure traceability of components.
Document manufacturing, sterilization, and packaging processes.
Train staff in device safety, compliance, and regulatory requirements.
Monitor suppliers and subcontractors for quality consistency.
Carry out internal audits and address nonconformities.
Review QMS performance through management oversight.
Demonstrate continual improvement across all lifecycle stages.
Tip: Always align ISO 13485 with ISO 14971 for risk management. This dual approach ensures compliance with both quality and safety requirements.
How to prepare for ISO 13485 certification?
Preparation involves aligning regulatory requirements with ISO standards to ensure smooth certification and faster market entry. Refer to the points below:
Conduct a gap analysis against ISO 13485 and regulatory frameworks (e.g., MDR, FDA).
Develop policies for design, manufacturing, and risk management.
Train staff in quality management, safety practices, and documentation.
Collect evidence such as validation reports, risk files, and supplier audits.
Test internal audits to confirm readiness.
Monitor KPIs like defect rates, CAPA closures, and audit findings.
Engage leadership to support compliance and resource allocation.
Certification audit
Stage 1 audit: Review of QMS scope, documentation, and risk files.
Stage 2 audit: Evaluation of implementation across design, manufacturing, and supply chains.
Nonconformities: Must be addressed with documented corrective actions.
Management review: Confirms leadership’s involvement in QMS.
Final certification: Awarded once requirements are met.
Surveillance audits: Conducted annually to ensure compliance.
Recertification audits: Every three years to maintain certification.
What are the benefits of ISO 13485 certification?
ISO 13485 certification creates strong advantages for medical device manufacturers and suppliers, improving safety, quality, and regulatory compliance. Below are the key benefits:
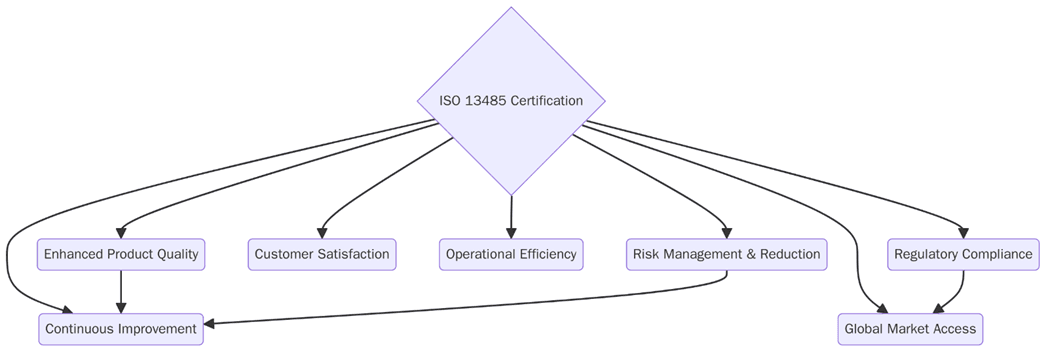
Greater access to global markets through recognition of compliance.
Improved product safety and patient outcomes through risk management.
Stronger reputation with regulators, buyers, and healthcare providers.
Lower recall rates and fewer product failures.
Increased efficiency in design, production, and supply chains.
In recent years, ISO 13485 is being widely adopted not just by large manufacturers but also by SMEs and component suppliers, driven by stricter MDR enforcement in Europe and FDA modernization efforts in the U.S. Digitalization is another trend, with QMS platforms being integrated to manage documentation, audits, and traceability more efficiently. Supply chain transparency is also a key focus, as buyers demand proof of compliance from all tiers of suppliers.
A McKinsey report projects that companies with ISO 13485 certification will continue to benefit significantly in the coming decade. By 2030, certified manufacturers are expected to see 20–30% faster approval times for new devices, as regulators streamline processes for organizations with proven compliance. Product recalls are anticipated to drop by more than 30% on average for certified companies, reducing both financial losses and reputational risks. The European Commission also forecasts that ISO 13485 will be a de facto requirement for over 70% of EU buyers by 2030, cementing its role not only as a quality standard but as a passport to global market access.
Training and courses
Pacific Certifications provides accredited training programs for ISO 13485 that help professionals and organizations understand, implement, and audit medical device quality management systems effectively:
Lead Auditor Training: Designed for professionals responsible for auditing and evaluating ISO 13485-based quality management systems within medical device manufacturing and supply environments.
Lead Implementer Training: Intended for personnel developing, maintaining, or enhancing ISO 13485 systems.
Contact [email protected] to schedule your ISO 13485 training or awareness session and build competency in medical device quality management.
How Pacific Certifications can help?
Pacific Certifications provides accredited ISO 13485 certification services for medical device manufacturers and suppliers. Our independent audits confirm compliance, strengthen credibility, and support global market access.
Request your ISO audit plan and fee estimate, we will help you map Stage 1 and Stage 2 timelines and evidence requirements for your organization. Contact us at [email protected] or visit www.pacificcert.com.
Contact Us
If you need support with ISO 13485 certification, contact us at [email protected].
Read More at: Blogs by Pacific Certifications
Read more: Pacific Blogs

