ISO 13485 Certification in California: Elevating Medical Device Manufacturing

Introduction
Medical device manufacturing is one of the most regulated sectors in which significant quality system and product requirements must be satisfied. Regulatory requirements are intended to ensure that manufacturers consistently design, produce and place onto the market, medical devices that are safe, and operate in accordance with their intended purpose. A medical device manufacturer’s quality management system (QMS) represents a solid foundation to maintain manufacturing and operational high-quality standards, while complying with regulatory requirements. A compliant QMS drives improvement and effectiveness, whilst promoting trust in the manufacturer and in the devices placed on the market, as well as on other processes involved in the medical device life-cycle, such as design, development, production, storage and distribution.
What is ISO 13485 Certification?
ISO 13485 is an internationally recognized standard that specifies requirements for a quality management system in organizations involved in the design, production, installation, and servicing of medical devices. The standard provides a structured framework that helps businesses ensure their products consistently meet regulatory requirements and are safe for their intended use.
While ISO 13485 is not legally required in every market, many regulatory authorities, such as the U.S. Food and Drug Administration (FDA), require medical device manufacturers to adhere to quality management standards like ISO 13485 as part of their approval process. Certification to this standard serves as evidence that a manufacturer has established a robust QMS that effectively controls the design, development, and production processes.
The importance of ISO 13485
ISO 13485 is critical to manufacturers but also to importers, suppliers and distributors to enhance their organisation’s trust and marketability, particularly as more and more manufacturers require ISO 13485 certification when outsourcing certain medical device related services. When it comes to medical device manufacturing, patient safety greatly depends on the quality and consistency of the QMS in which medical devices are manufactured. Ensuring effectiveness, control and maintenance of your QMS is critical to customers, stakeholders, patients, users and Competent Authorities. The value of ISO 13485 is not just in its implementation, but also in providing practical guidance on organisational internal processes for the assessment of your QMS effectiveness.
What are the Benefits of ISO 13485 Certification?
ISO 13485 certification offers numerous benefits to medical device manufacturers in California. These benefits include:
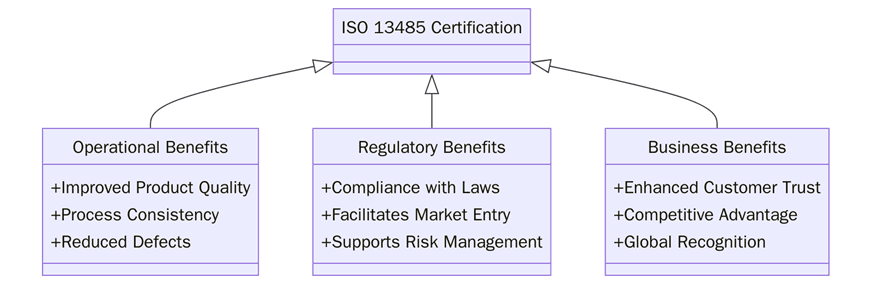
1. Being aware of or establishing ever-stricter quality controls in every phase of the product cycle enables manufacturers to always produce those devices that satisfy regulatory and customer requirements.
2. ISO 13485 ensures a manufacturer follows best practices and international standards for quality and safety, and that means a lot of reassurance for customers and regulators as well. It is also a benefit to a healthcare provider in his/her search for reliable and effective devices.
3. By catering to the structured requirements of QMS as specified in this ISO 13485, companies can internal processes, cut down on wastage, and proceed with a general enhancement toward improvement. The standard calls for continual improvement, which keeps organizations on their toes as the market changes from time to time.
4. Because of its focus on risk management and optimization of processes, ISO 13485 helps manufacturers with minimizing the risks of product defects, recalls, and complaints from customers. That is what eventually brings down the costs, thereby enhancing profits.
5. ISO 13485 certification is internationally recognized; thus, California-manufactured product obtains recognition in international markets, thereby fulfilling the demands of global regulators and customers across the globe.
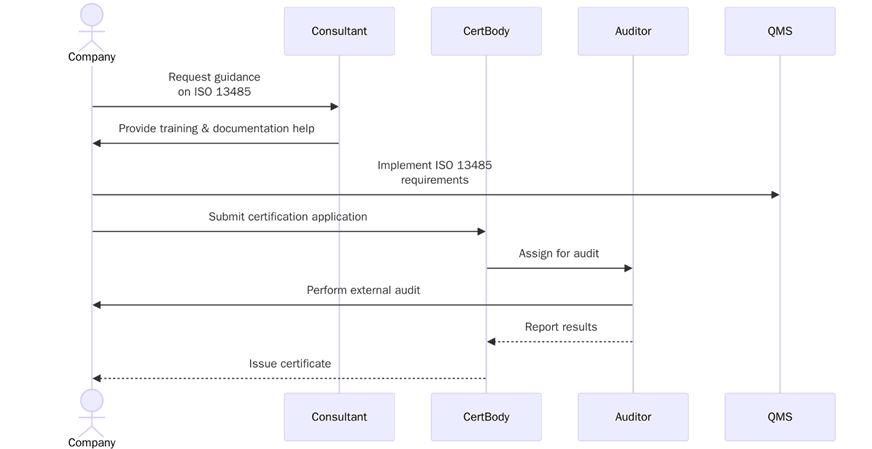
How to Obtain ISO 13485 Certification in California?
The process of obtaining ISO 13485 certification typically involves several steps, from initial preparation to ongoing maintenance of the QMS. Here's a breakdown of the general steps involved:
1. Understanding the Requirements
The initial stage in the certification process is to understand ISO 13485 requirements and how they apply to your organization. Get acquainted with key clauses and processes: risk management, document control, internal audits, and regulatory requirements.
2. Developing and Implementing a QMS
Next, you need to develop and implement a QMS in accordance with the ISO 13485 standard. This should include processes related to product design, production, testing, and post-market surveillance. The QMS should also contain procedures on quality control, document management, and continual improvements.
3. Training Your Team
The process of becoming ISO 13485 certified is wholly dependent on all personnel within the organization. Everyone has to be trained on quality management principles and processes as described within ISO 13485, so that everyone is working towards the same quality and safety goals.
4. Conducting Internal Audits
Once QMS is established, internal audits need to be conducted to verify that processes are working as intended. These audits expose gaps and areas for improvements before the certification body can come for the official audit.
5. Select a Certification Body
Select an accredited certification body to do the methodical ISO 13485 correspondence audit. The certification body will evaluate your QMS, assess your compliance with ISO 13485, and confirm that activities carried out by your organization's actions conform to ISO 13485.
6. Keep and Revise Your QMS
Once you've received certification, you will want to keep and improve your QMS to ensure that you maintain compliance with ISO 13485. Auditing, training, and consistency reviews will support your compliance process remain effective and keep pace with shifting regulations.
Contact Us
For assistance with ISO 13485 certification in California, Pacific Certifications is here to guide you through the process. Our team of experts can help you implement a quality management system that meets the highest standards and ensures compliance with regulatory requirements.
Contact Details:
Email:[email protected]
Website:www.pacificcert.com
Our certification services will help you establish a robust QMS that enhances product quality, reduces risks, and ensures long-term success in the medical device industry.
Contact Us
If you need support with ISO certifications in US, contact us at [email protected].
Read More at: Blogs by Pacific Certifications

