IEC 62304:2006 – Medical Device Software — Software Life Cycle Processes

Introduction
Software is often an integral part of medical device technology. Establishing the safety and effectiveness of a medical device containing software requires knowledge of what the software is intended to do and demonstration that the use of the software fulfils those intentions without causing any unacceptable risks.
This standard provides a framework of life cycle processes with activities and tasks necessary for the safe design and maintenance of medical device software. This standard provides requirements for each life cycle process. Each life cycle process is further divided into a set of activities, with most activities further divided into a set of tasks.
As a basic foundation it is assumed that medical device software is developed and maintained within a quality management system and a risk management system. The risk management process is already very well addressed by the International Standard ISO 14971. Therefore IEC 62304 makes use of this advantage simply by a normative reference to ISO 14971. Some minor additional risk management requirements are needed for software, especially in the area of identification of contributing software factors related to hazards.
What is IEC 62304:2006?
IEC 62304 is an international standard that defines the life cycle processes for software used in medical devices. It provides a framework for the entire software development life cycle, from the initial concept and planning phase to post-market maintenance. The standard outlines the necessary processes for software development, risk management, testing, and maintenance, ensuring that software meets safety, reliability, and regulatory requirements.
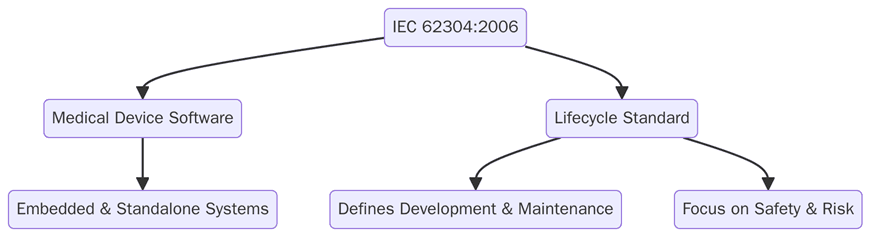
The standard is applicable to all medical device software, regardless of complexity or functionality, and can be used by manufacturers of both Class I, II, and III devices as well as combination products that include software.
What are the requirements of IEC 62304?
IEC 62304:2006 outlines a comprehensive set of life cycle processes for software in medical devices. These processes are divided into several phases, each with its specific requirements and objectives.
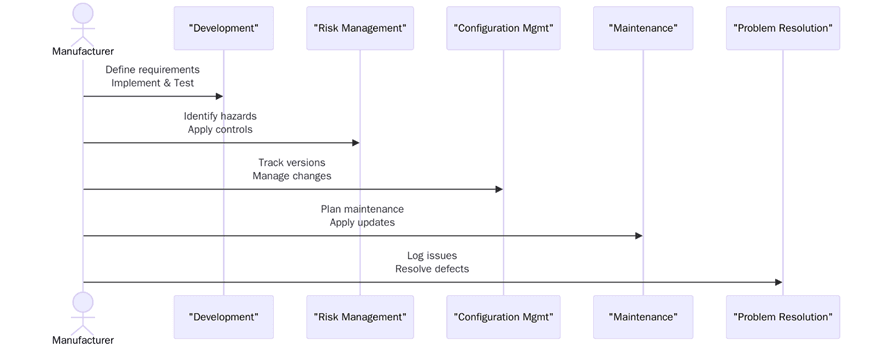
1. According to the medical device software development life cycle, there are different phases through which the software passes: software planning, design, implementation, testing, releasing, and maintenance. The software planning phase starts with setting the requirements for software, the scope, resources, and schedules. Besides the above, establishment of a risk management plan must also be included.
2. Risk management comes as the heart of IEC 62304. This standard explains that risks related to software are to be identified, analysed, and lessened during the entire lifecycle of software development. Hence, an organization has a duty to take into account possible hazards and the likelihood of their occurrence, evaluate the potential consequences of the software failures, and that of impact on patient safety. The standard codifies an approach to risk management so that controls could be implemented for reducing risks to an acceptable level.
3. Configuration management is concerned with the management and control of changes to software and associated documents. In IEC 62304, configuration management shall ensure that all changes of the software are recorded, tested, and documented. The aim is to maintain the integrity and traceability of the software during the software life-cycle. It involves exercising controls on versioning, change tracking, and ensuring all relevant stakeholders have been informed of the changes made to the software or the hardware on which the software is operated.
4. Software maintenance is the other requirement of IEC 62304, reaching beyond the initial release of the software. Medical device software needs to be maintained through its life cycle to ensure that it remains in compliance with regulatory requirements and the latest safety standards. Maintenance activities include post-market surveillance, wherein the performance of the software is observed for any undesirable consequences in the real-world scenarios between the distributors and the users of the software under clinical settings.
Benefits of IEC 62304:2006 for Medical Device Manufacturers
IEC 62304:2006 offers several benefits to manufacturers of medical device software, helping them develop safe, reliable, and compliant software systems:
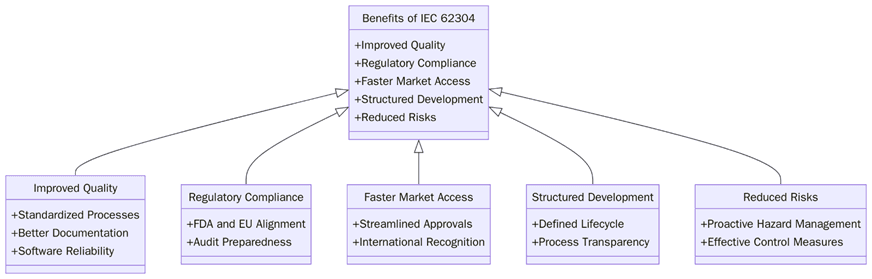
Regulatory bodies such as the FDA and EMA require medical device manufacturers to follow a structured approach to software development. By complying with IEC 62304:2006, manufacturers can meet the software-related regulatory requirements for medical devices, facilitating the approval process in various markets.
IEC 62304:2006 emphasizes rigorous software testing, validation, and risk management, ensuring that medical device software is of the highest quality. This helps manufacturers identify and mitigate risks early in the development process, reducing the likelihood of software failures that could compromise patient safety.
By following the guidelines outlined in IEC 62304:2006, manufacturers can ensure that they meet all necessary quality and safety requirements for medical device software. This reduces the risk of non-compliance, product recalls, and penalties due to software-related issues.
IEC 62304:2006 requires robust documentation of the software development process, from planning and design to maintenance and post-market surveillance. This enhances traceability, ensuring that all changes, updates, and decisions are recorded and can be reviewed by regulators if necessary.
The structured approach provided by IEC 62304:2006 helps streamline the software development process, ensuring that all necessary steps are followed and that software is delivered on time and within budget.
Contact Us
For guidance on implementing IEC 62304:2006 and ensuring compliance with software life cycle requirements for medical devices, Pacific Certifications is here to help. Our team of experts can assist you with developing, testing, and maintaining medical device software that meets the highest safety and regulatory standards.
Contact Details:
Email: [email protected]
Website: www.pacificcert.com
We provide certification services that help manufacturers navigate the complexities of medical device software development, ensuring compliance and safety at every stage of the software life cycle.
Contact Us
If you need support with IEC 62304:2006, contact us at [email protected].
Read More at: Blogs by Pacific Certifications

